Try It
To try out your own listening skills, pick a sample of student thinking from the list below. (Tip: We’re often better at listening in disciplines besides our own.)
Try to put aside questions of whether the student is right or wrong, and just try to answer the questions: What is this student’s idea? What is the evidence for your interpretation?
Instructional context
- In-person quiz in Developmental Biology - an upper-level course offered by the Department of Biology
Disciplinary background
- Induced pluripotent stem cells (iPSCs) are cells made by exposing specialized cells— such as epithelial cells, nerve cells, muscle cells, or fibroblasts— to chemicals that turn the cells into a less specialized state.
- iPSCs can replicate and can become any type of body cell.
Original prompt
Can medical treatments using induced pluripotent stem cells (iPSCs) derived from a patient’s own fibroblasts be potentially harmful to the patient? In a few sentences please provide a rationale for your answer.
Vicky's response

Daren's response

- self-antigen: chemicals on a cell's surface that are recognized by an organism's immune system as "self", causing the immune system to spare the cells from destruction
- extracellular matrix (ECM): a fibrous material that cells make and release around themselves
Instructions for listening
Try to put aside questions of whether the student is right or wrong, and just try to answer the questions: What is this student’s idea? Try to pay attention to what there's actually evidence for as opposed to assumptions you may be making.
Instructional context
- Take-home quiz in Chemical Principles 2 - the second course in a series of an introductory courses offered by the Department of Chemistry and taken by students intending to major in one of a variety of majors, including the natural sciences and engineering
Original prompt
Do you think the atomic radius for H in HCl versus H in hydrochloric acid in aqueous solution would be the same? Explain briefly.
| Rashied | I think that when HCl is in an aqueous solution, hydrogen will have a smaller atomic radius, because it loses one electron to chlorine. This would cause the atomic radius to be smaller because there are less electrons in orbit and hydrogen now has a +1 charge, placing more charge on the positive protons in the nucleus of the atom. In pure hydrochloric acid hydrogen does not lose an electron and will have a bigger atomic radius. |
| Alice | The H in HCl would not be the same as the H in hydrochloric acid in aqueous solution because the dissociated H in the aqueous solution would turn into H+ making it smaller than the H+ in HCl. The H+ is smaller than the neutral H because it has the same number of protons with one less electron needing to be pulled in from the protons in the nucleus. The dissociated H+ ion in the aqueous solution is smaller than the H+ in HCl because H+ in HCl is sharing the electron with Cl- through an ionic bond while the dissociated H+ does not have to do that. |
| Warner | No, I think the atomic radii would be different. In HCl, the H and Cl share valence electrons that spend more time around Cl, resulting in a larger atomic radius for H. In comparison, hydrochloric acid dissociates in water, leaving the H atom to bond with the water molecule to form H3O+. Since H3O+ is an ion with a positive charge, it has more protons than electrons, which means that the protons are able to attract the electrons more and bring them closer together. Thus, the H in hydrochloric acid in aqueous solution has a smaller atomic radius. |
| Diana | No because in non-aqueous HCl the hydrogen atom is interacting directly with the chloride, whereas the water molecules in the aqueous solution would be in-between the ions and suspending them. My prediction is that the radius of the hydrogen ion is smaller in the non-aqueous form because it's existing in an ionic bond and it's larger in the solution because the intermolecular forces of the negatively charged portion of the water molecule is not pulling as strongly. |
| Sy | H in HCl will have a smaller atomic radius than H in hydrochloric acid in aqueous solution because in the solution H and Cl disassociate so they are not bonded by a covalent bond together. The covalent bond in HCl shortens the radius. |
| Camile | Yes, because in each scenario, the H has donated its electron to Cl. |
| Sacha | Yes it would be the same, the makeup of each HCl atom hasn't changed, they are just mixed with H2O atoms. |
| Henry | Yes, I think it would be the same. The intermolecular forces change in a solution because the HCl spreads out more, but the intramolecular forces that keep H bonded to Cl shouldn't change. |
Instructions for listening
Try to put aside questions of whether the student is right or wrong, and just try to answer the questions: What is this student’s idea? Try to pay attention to what there's actually evidence for as opposed to assumptions you may be making.
Instructional context
- General Physics 1 - an introductory course taken mainly by students intending to major in physical science or engineering
- Whole-class discussion following a clicker question and time for students to talk with each other in small groups
Original prompt
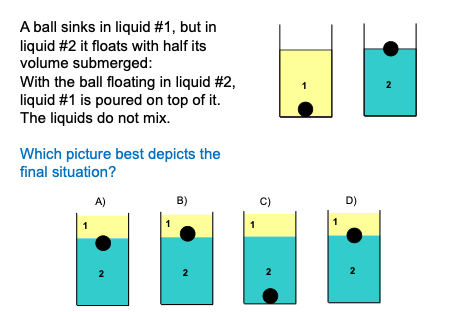
| Abner | The way I thought about it was, um, when liquid 1 is poured on top of liquid 2 and the ball, [INSTRUCTOR: OK] because it sinks in liquid 1, uh, liquid 1 puts, uh, the same... it kinda, it kinda puts a downward force due to the weight of it and due to the pressure of it [INSTRUCTOR: Mmhm.] that, um, exceeds the buoyant force the ball feels when its touching the liquid, so it sinks further down, so I voted for A. |
| Bob | Uh, I I voted B. [INSTRUCTOR: OK] Uh, I thought because, the wa—the uh, liquid 1 is on top of liquid 2, so its exerting like a greater pressure, and so it’s gonna be deeper— so all of the water is going to be deeper so the pressure at the surface is gonna increase, and so the buoyant force will increase, on the ball, so it’s going to be raised up a little bit. |
| Celia | Um so so I think about how maybe liquid 1 will push it down, at the same time liquid 2 is going to push it up so in my opinion it’s going to end up staying the same because because there’s going to be force () on the top of the ball () |
| Douglas | But that’s dependent on the density of each fluid and the volume displaced of each fluid, so I find it hard to believe that those forces can exactly counteract, given that it sinks in fluid 1 and floats in fluid 2. |
| Ezra | Well, if we look at, um, example 2, there’s actually two fluids—there’s air and there’s, um, liquid 2. And I think it’s safe to assume that, uh, 1 is more dense than air, so if you look at 1 as if it was air, then it would push further down onto than air, so I say it’s A. |
| Fred | But uh, on that line, if its more dense than air, wouldn’t it provide more buoyant force upwards? 'Cuz you—'cuz it weights more than air? |
Instructions for listening
Try to put aside questions of whether the student is right or wrong, and just try to answer the questions: What is this student’s idea? Try to pay attention to what there's actually evidence for as opposed to assumptions you may be making.

The Listening Project is supported by a grant to Tufts University from the Howard Hughes Medical Institute through the Science Education Program.